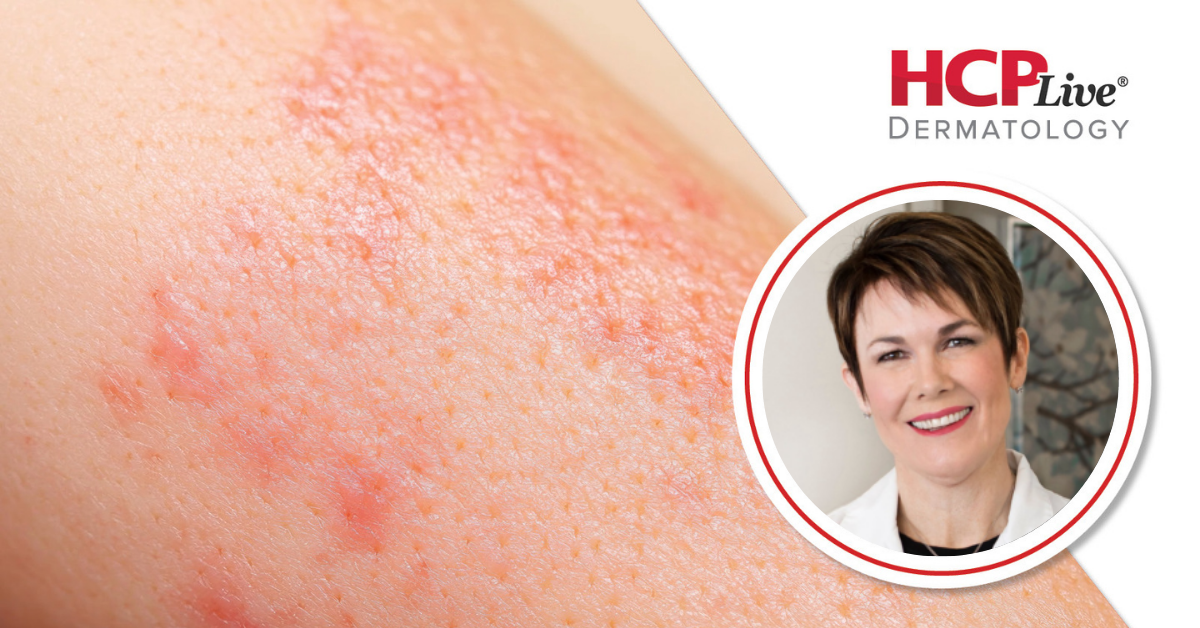
Takeda Pharmaceuticals announced positive topline results from two pivotal phase 3 studies on its oral medication, zasocitinib (TAK-279), for adults suffering from moderate-to-severe plaque psoriasis. Released on December 18, 2023, the findings indicate that zasocitinib significantly outperformed placebo in achieving co-primary endpoints, which include the static Physician Global Assessment (sPGA) 0/1 and the Psoriasis Area and Severity Index (PASI) 75 scores.
To delve deeper into the implications of these results, HCPLive spoke with Melinda Gooderham, MD, a dermatologist and medical director at the SKiN Centre for Dermatology in Ontario, Canada. She described the studies, known as the Latitude studies, as two randomized, multicenter, double-blind trials that included both placebo and active comparator controls. Conducted across 21 countries, the studies enrolled 693 and 1,108 participants, respectively.
The co-primary endpoints were evaluated at the 16-week mark, focusing on the proportion of patients achieving sPGA 0/1 and PASI 75 responses. Gooderham noted that the results revealed substantial efficacy for zasocitinib, with a significant number of PASI 75 responses occurring as early as 4 weeks into the treatment and continuing to increase through to Week 24.
In the announcement, Takeda stated that all 44 ranked secondary endpoints were met, including sPGA 0, PASI 90, and PASI 100, demonstrating the drug’s effectiveness compared to both placebo and apremilast. Gooderham emphasized the significance of having a once-daily oral treatment that offers the potential for complete skin clearance in psoriasis patients.
“I think what’s exciting is that we’re seeing these levels of biologic efficacy,” Gooderham commented. “In the past, there was often a trade-off with our oral therapies being less effective than biologics.”
Regarding safety, the drug was generally well tolerated, aligning with findings from earlier studies. The most frequently reported adverse events during the 24-week study period included upper respiratory tract infections, acne, and nasopharyngitis. Importantly, no new safety signals were identified, ensuring ongoing confidence in the medication’s profile.
Takeda plans to present detailed findings at upcoming medical congresses and intends to submit a New Drug Application to the U.S. Food and Drug Administration (FDA) and other regulatory authorities by 2026.
Gooderham has disclosed nonfinancial support from Takeda and personal fees from various pharmaceutical companies, including AbbVie and Amgen, among others. These disclosures highlight her engagement with the industry while discussing the potential impact of zasocitinib on psoriasis treatment.
As the medical community anticipates further insights from these studies, the results underscore a significant advancement in the management of psoriasis, potentially transforming the treatment landscape for affected individuals.