A research team at the University of Adelaide has developed a groundbreaking approach to enhance the performance of aqueous zinc-iodine batteries, a potential alternative to traditional lithium-ion energy storage systems. This innovation addresses significant challenges associated with self-discharge rates, which can undermine the efficiency of these batteries in large-scale applications.
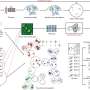
Led by Professor Shizhang Qiao, Chair of Nanotechnology at the School of Chemical Engineering, the team explored the use of ferrocene in cathodes to improve battery performance. Their findings were published in the esteemed journal Nature Chemistry. The conventional iodine cathodes in zinc-iodine batteries often suffer from slow reactions and inconsistent electrochemical performance, prompting the researchers to investigate ferrocene as a solution.
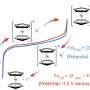
Professor Qiao explained, “The conversion of iodine in aqueous zinc-iodine batteries accompanies the polyiodides shuttle effect. However, the conversion of ferrocene can precipitate the polyiodides, which results in a low self-discharge.” This crucial insight indicates that ferrocene not only enhances the battery’s performance but also contributes to its cost-effectiveness due to its composition of low-cost elements.
The research highlights that integrating ferrocene into the battery system can reduce overall costs by 9% compared to systems that do not utilize this organometallic compound. By effectively eliminating the shuttle effect—a common issue in zinc-iodine batteries where intermediate polyiodides dissolve in the electrolyte and cycle between the cathode and anode—this advancement represents a significant step forward in battery technology.
In addition to improving energy density, the use of ferrocene offers a practical and scalable strategy for the development of aqueous zinc-iodine batteries. Professor Qiao emphasized the broader implications of their work. “Our findings also show that the active mass in the cathode can reach 88%, minimizing capacity loss from inactive hosts.”
The ongoing research at the Center for Materials in Energy and Catalysis signifies a promising future for sustainable energy storage solutions. With the potential for lower production costs and improved efficiency, aqueous zinc-iodine batteries could play a pivotal role in meeting the growing demand for large-scale energy storage systems.
As the world increasingly turns to renewable energy, innovations like those from the University of Adelaide may help facilitate a smoother transition towards greener technologies. This research not only showcases the power of scientific inquiry but also highlights the importance of developing energy systems that are both economical and environmentally friendly.