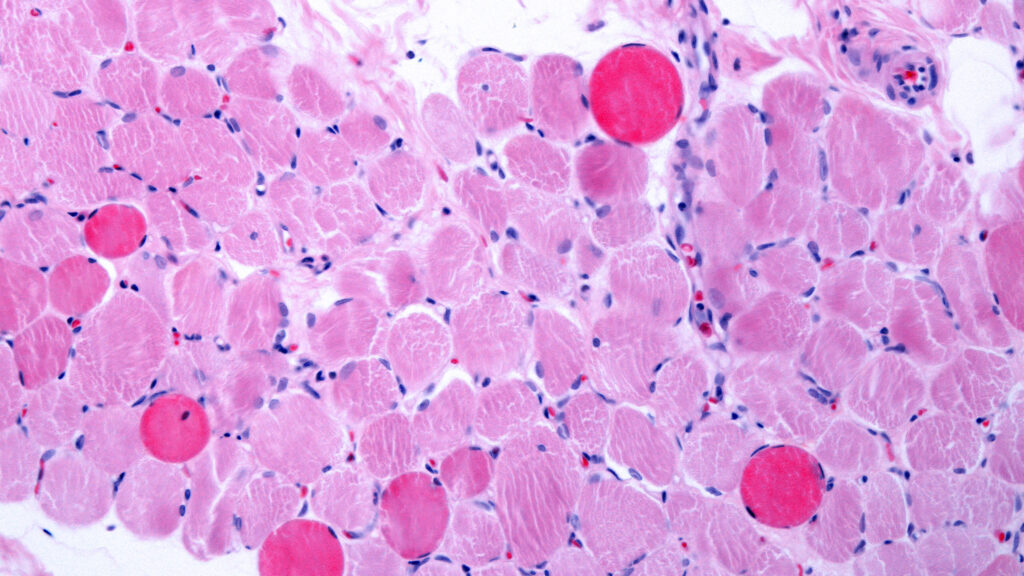
Capricor Therapeutics announced on Wednesday that its cell therapy, known as deramiocel, has significantly improved heart and muscle function in patients with Duchenne muscular dystrophy (DMD). This outcome, achieved in a Phase 3 clinical trial, represents a critical advancement for the treatment and could influence its ongoing regulatory review in the United States.
The results from this latest study indicate marked improvements in both muscle strength and cardiac function, which were identified as the primary endpoints of the trial. These findings may strengthen Capricor’s case as it seeks to overturn a previous decision made by the Food and Drug Administration (FDA). In July 2023, the agency rejected Capricor’s application for deramiocel, citing insufficient evidence of the therapy’s effectiveness, a ruling that was reportedly influenced by Vinay Prasad, the FDA’s leading regulator for cell and gene therapies.
Capricor’s CEO, Linda Marban, expressed optimism about the new results. She believes they provide compelling evidence that could lead the FDA to reassess its earlier stance. Marban highlighted the significance of the larger, placebo-controlled study, which she argues demonstrates the therapy’s potential benefits more convincingly than previous trials.
The Phase 3 study was designed to evaluate the safety and efficacy of deramiocel in a larger patient population, aiming to address the concerns raised by the FDA in its prior review. DMD is a rare and severe genetic disorder characterized by progressive muscle degeneration, and the need for effective treatments is urgent.
As Capricor prepares to submit this new data to the FDA, the company faces both hope and scrutiny. The regulatory landscape for cell therapies is complex and often contentious, as the FDA aims to balance innovation with patient safety. Should the agency reconsider its previous decision, deramiocel could become a groundbreaking option for those affected by DMD.
The path ahead remains uncertain, but with promising results now on the table, Capricor’s future looks more optimistic. The company is committed to advancing its research and engaging with regulatory bodies to ensure that this potential therapy reaches the patients who need it most.